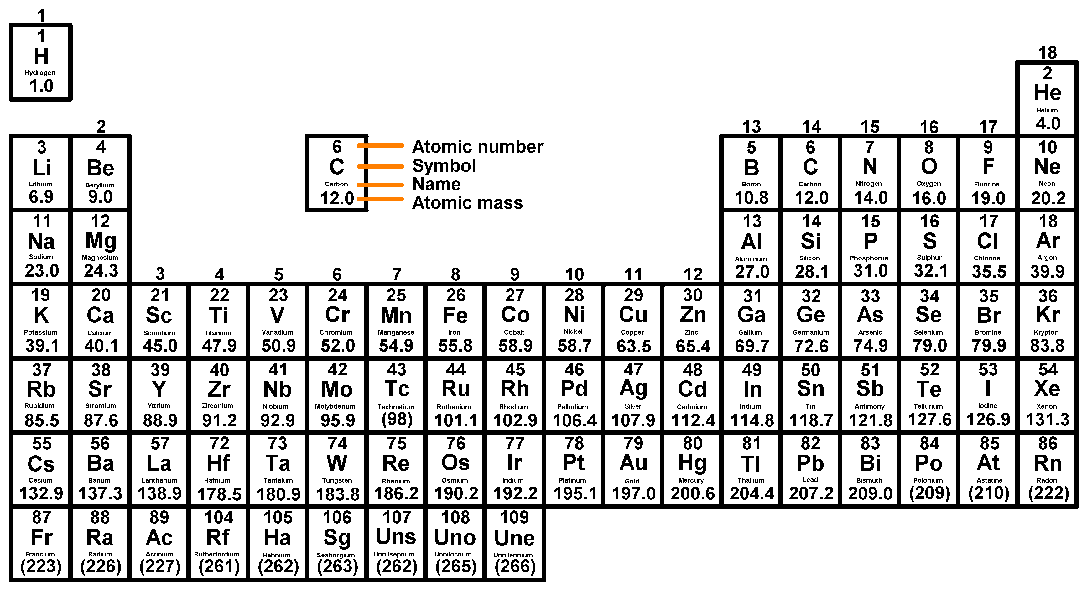
- What information the Periodic Table tells us.
- The definition of element, proton number and mass number.
- The arrangement of the Periodic Table.
- The fundamental particle the Periodic Table is based on.
Notes:
- The periodic table is an organized list of all the different types of atoms known to scientists.
- We call each different type of atom an element. Whenever you read 'element' in chemistry, the reading is talking about a unique type of atom.
- The difference between any one element and another is the number of protons that an atom of that element contains. For example, carbon atoms by definition always contain 6 protons each. If an atom doesn't have 6 protons, it is not a carbon atom. If an atom does have 6 protons, it is a carbon atom – no exceptions!
- The periodic table arranges all known elements by the number of protons they contain. This is known as their atomic number or their proton number. It runs from smallest proton number in the top-left corner (Hydrogen, which has atoms of only one proton) to the bottom-right (where the atoms of some elements have over 100 protons)
- The periodic table is also arranged in groups which run vertically (the columns of the table), and periods which run horizontally (the rows of the table). For example, all of the elements in the first column on the left of the table are called group 1 elements. We think of the elements in the same column as in a group because by experimenting, chemists can show the elements in the same columns have a lot in common.
- For each element, the entry in the periodic table shows its chemical symbol, its atomic mass (AKA its mass number) which is the larger number, and its atomic number (AKA its proton number) which is the smaller number.
- As said above, the defining difference between any two elements is the number of protons its atoms contain. A proton is a particle contained within the atom that affects the properties of elements. We'll learn more about protons in our structure of the atom lesson!