- The definition and general formula of an alcohol with some basic examples.
- The major properties of alcohols and their difference to the hydrocarbons.
- The reactions of alcohols and how the type of alcohols affects reactivity.
- How to name alcohols using IUPAC organic nomenclature.
Notes:
- Alcohols are another homologous series: a "family" of organic molecules with the same general formula, each member differing from the next by a -CH2- hydrocarbon chain unit.
Any organic compound where the hydroxyl (-OH) group is covalently bonded to a saturated carbon atom is an alcohol.
The -OH hydroxyl group is the functional group that makes an alcohol react the way it does. A molecule must have a carbon atom with four single bonds, one to an OH group, to be called an alcohol. - Unlike alkanes, alkenes and alkynes, alcohols are not hydrocarbons. They are not considered hydrocarbons because alcohols contain an oxygen atom in the molecule.
- Alkanes have the general formula: CnH2n+2O but it is normally written as CnH2n+1OH, to show the hydroxyl group that defines an alcohol. Alcohols then are very similar to alkanes of the same carbon chain length; there is same number of carbons and hydrogens, just with an extra oxygen atom.
This is because a hydroxyl group is just an oxygen atom bonded to a hydrogen atom, making one bond to the rest of the molecule through a carbon atom. Naming of simple alcohols is very similar to alkanes as a result; alcohols have the suffix ol instead of -e in alkanes and alkenes. See the table below for the names of some simple alcohols. - Alcohols are important solvents in chemistry. They are used to dissolve other chemicals. The most generally used alcohol is ethanol, CHOH which is used as a fuel and is the alcohol used in alcoholic drinks.
- Despite being quite similar in structure to basic alkanes, the properties of alcohols are very different from alkanes. The OH group means hydrogen bonding exists between separate alcohol molecules, which affects their melting/boiling points and solubility. Oxygen is a very electronegative atom with two lone pairs, which the hydrogen atom of OH groups in other molecules can interact with.
The major properties of alcohols are: - A much higher melting and boiling point than the alkanes of the same chain length. Methanes boiling point is around -164°C, while methanols boiling point is around 60°C.
- Polarity: shorter alcohols are polar molecules that dissolve in water, not fats. The longer the chain, the less mixable with water they are.
- Toxic: all alcohols are toxic, including ethanol found in alcoholic drinks (drunkenness is also known as intoxication!).
- Flammable: alcohols burn with a relatively clean flame compared to alkanes and do not produce soot (carbon particulates).
- There are clear trends in their properties. These property trends can be explained by intermolecular forces. As the carbon chain length of alcohols gets longer, they:
- Have a higher melting/boiling point. As the carbon chain gets longer there is yet more van der Waals around it as other carbon chains can align with it, so MP/BP increases accordingly.
- Are less volatile / flammable. The longer chain alcohols have a lower tendency to vaporize with greater IMFs between the molecules.
- Are less soluble in water. The longer carbon chain makes the molecule more hydrophobic in general, so they do not mix with water as well as the shorter alcohols. Ethanol is highly soluble in water but hexanol is not.
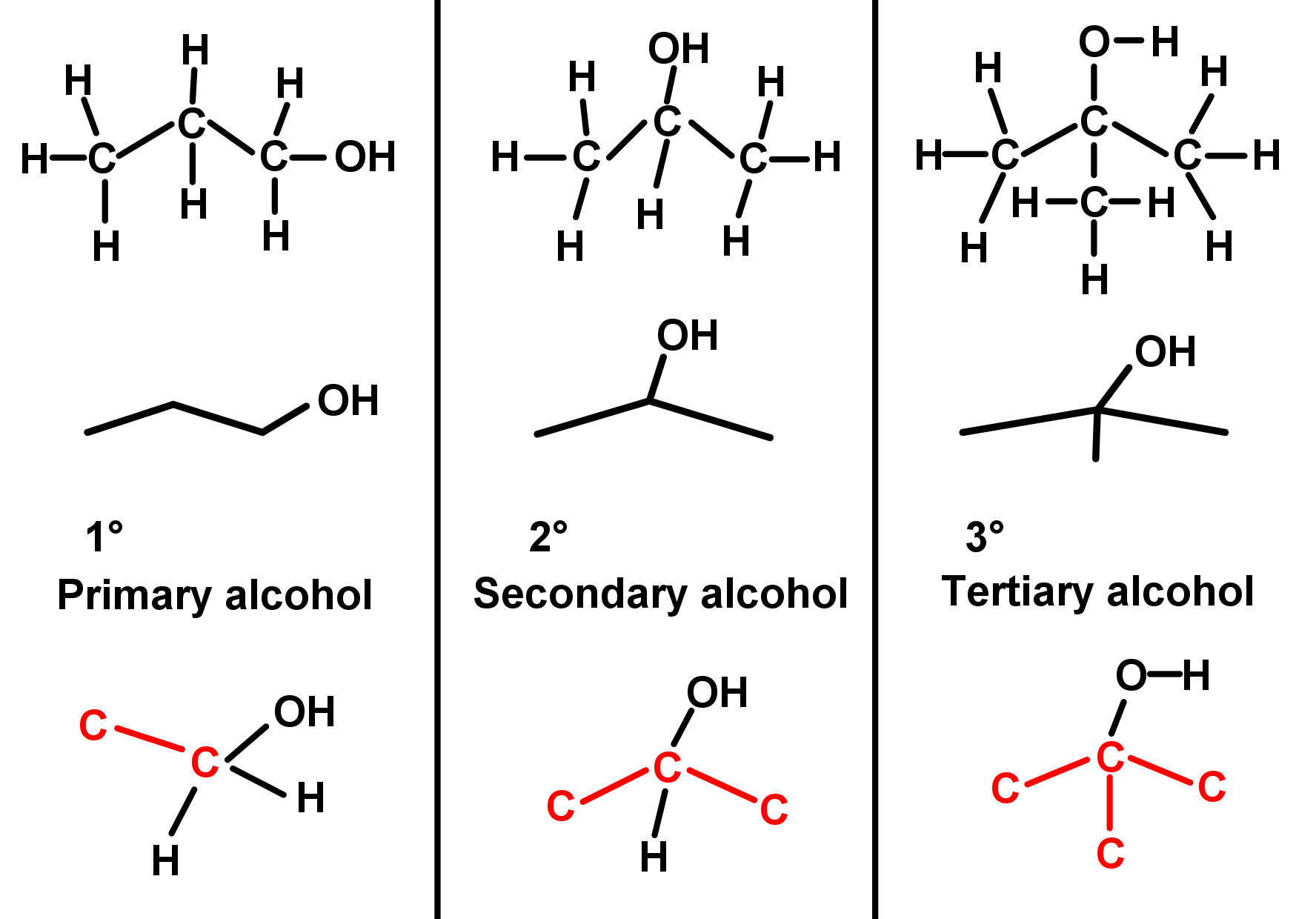
- Depending on the carbon atom the hydroxyl OH group is bonded to, we can describe three types of alcohols. There are also chemical tests to determine which one we have. Testing for the presence of alcohol compounds involves using acidified potassium dichromate.
- A primary alcohol (1°) is an alcohol with the –OH group bonded to a carbon atom making only one carbon-carbon bond. This would place the –OH group at the end of a carbon chain.
- A secondary alcohol (2°) is an alcohol with the –OH bonded to a carbon atom making two carbon-carbon bonds. This would place the –OH group in the middle of a carbon chain.
- A tertiary alcohol (3°) is an alcohol where the –OH group bonds to a carbon atom with three carbon-carbon bonds. This would place the –OH group on a carbon where a branch in the carbon chain is found. See the table (the brackets in the middle column show branching) and examples.
- Alcohols are known to perform a number of reactions:
- Alcohols react with alkali metals to form metal salts and hydrogen gas. The reaction with sodium is:
- Alcohols also react with acids to form alkyl halides and water as a side product. The reaction with hydrochloric acid is:
- As mentioned above, alcohols can be burned easily like alkanes and alkenes, but they burn more cleanly. This means more complete combustion occurs so less soot (carbon particulates) and CO is made. For example, with ethanol:
- Alcohols can be oxidized into aldehydes or ketones, depending on the type.
We usually represent the oxidizing agent with an [O] in the chemical equation, so the focus stays on the organic substance. [O] just means it supplies oxygen to the other substance. Primary alcohols will be oxidized to aldehydes, and secondary alcohols will be oxidized to ketones. Tertiary alcohols cannot be oxidized. The oxidation of ethanol to ethanol is shown below: - Primary alcohols can be oxidized straight to carboxylic acids, skipping the aldehyde step in between. Remember that ketones cannot be reacted to make carboxylic acids, only aldehydes can. The full oxidation of ethanol is below:
- In most cases when the aldehyde is produced, it will react further and make the carboxylic acid in the last equation above. For this reason, the reaction is often done under reflux with distillation to separate the two products the aldehyde and the carboxylic acid.
- To name alcohols using IUPAC nomenclature, the –OH group is given the suffix –ol. It is also given a number to show which carbon atom in the main chain it is bonded to.
- All the systematic rules of naming alkanes, alkyl branches and alkenes apply.
- Alcohol groups are higher order (priority) than alkenes and alkyl branches, so numbering prioritizes alcohol groups.
|
Carbon chain length |
Name of alcohol |
Molecular formula |
|
1 |
Methanol |
CH3OH |
|
2 |
Ethanol |
C2H5OH |
|
3 |
Propanol |
C3H7OH |
|
4 |
Butanol |
C4H9OH |
|
Type of alcohol |
C-OH bonding: |
Test to identify using: 1. Acidified potassium dichromate. 2. Acidified silver nitrate to the product of 1. |
|
Primary (1o) |
-CH2OH |
1. Solution changes colour from orange to green. 2. Silver mirror is observed in the test tube. |
|
Secondary (2o) |
-CH(OH)- |
1. Solution changes colour from orange to green. 2. Add acidified silver nitrate – no observed change. |
|
Tertiary (3o) |
-C(C)(OH)- |
1. No observed change. Orange solution stays orange. |